PHYSIOCHEMICAL PROPERTIES OF DRUG
Link for video explaination of Refractive index👇
https://youtu.be/PVJsBvEJtC4
And for Pulfrich Refractometer, factors and Applications👇👇👇
https://youtu.be/ChUfeKGzv1g
For Dielectric constant 👇👇👇
https://youtu.be/SwHapwn81QY
For Dipole moment 👇👇👇
https://youtu.be/eZeP23MAlXw
For Dissociation constant 👇👇👇
https://youtu.be/ZMqu_g-4Txk
For Optical rotation 👇👇👇
https://youtu.be/cYwJeLTQTno
INTRODUCTION
The study of physical and chemical properties of drug molecules is early requirement in preformulation of any product. It provide better understanding of inter relationship between molecular structure and drug action. These properties are additive (i.e., they are derived from sum of properties of individual atoms or functional groups within the molecule) as well as constitutive (depend on structural arrangement of atoms within the molecules). The physical properties of drug molecules, along with simple chemical derivation and degradation reactions, play an important part in the design of analytical methods.
REFRACTIVE INDEX
The refractive index or index of refraction (n) is one of the physicochemical properties of the substance. The refractive index is a dimensionless physical quantity specific to a certain medium. It is measure of speed of light which is equal to the velocity of light of a given wavelength in the empty space or in the vacuum divided by its velocity in the selected medium. It is expressed as:
n= c/v
Where
C= velocity of light of a given wavelength in vacuum
V= velocity of light of a given wavelength in selected medium
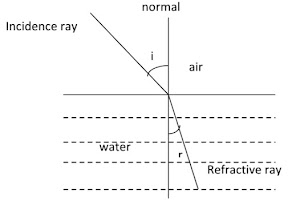
When a ray of light passes from air into a liquid , its direction is changed. This change of direction is called refraction.
Snell’s law describes the relation between the angles of incidence and refraction and may be written as:
n= sin i/ sin r
Where
i is the angle of incidence and
r the angle of refraction
According to the wave theory of light, the ratio of the sin of angles of incidence and refraction is identical to the ratio of the velocities of light in two media. Thus,
n= sin i/ sin r = velocity in air/ velocity in liquid
When a ray of light passes from a rare to denser medium, the law of refraction is shown as
sin i / sin r = n2/n1
Where
n1 is the index of refraction of the rare medium
n2 the index of refraction of the denser medium.
The angle of incidence can never be greater then 90° and when it is 90° (sin90°=1), the above equation will be shown as:
sin r = n1/n2
MEASUREMENT
The refractive index of different substrates measure with refractometers. There are different types of refractometer.
- Abbe’s Refractometer
- Pulfrich Refractometer
- Immersion Refractometer
- V block Refractometer
Principle: The Abbe’s refractometer is used to measure the refractive index of a liquid. The working principle is based on the measurement of the critical angle. The light from a radiation source is reflected by a mirror and hits a double prism. A few drops of the sample are placed between this so-called Abbe double prism. The incident light beams pass through the double prism and sample only if their angles of incidence at the interface are less than the critical angle of total reflection. A microscope and a mirror with a suitable mechanism are used to determine the light / dark boundary line (shadow line).
Working: The sample is contained as a thin layer (~0.1mm) between two prisms. The upper prism is firmly mounted on a bearing that allows its rotation by means of the side arm. The lower prism is hinged to the upper to permit separation for cleaning and for introduction of the sample. A sodium lamp is projected through the illuminating prism, whose lower surface is ground or roughened, so that each point on this surface generates light rays in all directions, when light is reflected into the prism, this surface effectively becomes the source for an infinite number of rays that pass through the sample at all angels.
The radiation is refracted at the interface of the sample and the smooth surface of the upper prism. After that, it passes into fixed telescope. The refractive index of a substance is a function of a wavelength. If the light source is not monochromatic, the light is scattered and the shadow boundary is not well defined. Instead of seeing a sharp edge between white and black, you will see a blue or red blurred border. In most cases, the measures are very inaccurate or even impossible. Therefore, to avoid dispersion, Abbe added two compensating Amiciprisms. The position of the Amici prisms can be adjusted to correct dispersion. Two Amici prisms serve to collect the divergent critical angle rays of different colors into a single white beam. A telescope is rigidly fixed in the sector which is fixed to upright of base so that the axis coincides with the rotation of the prism lens. The eyepiece of the telescope is provided with crosshairs: in making a measurement, the prism angle is changed until the light-dark interface just coincides with the crosshairs.
The position of the prism is then established from the fixed scale. Thermosetting is accomplished by circulation of water through the jackets surrounding the prism.
The Abbe's refractometer is very popular and owes its popularity to its convenience and to the minimal sample is needed.
Principle: Pulfrich refractometer is the second design (after Abbé refractometer) that became commercially available in 19th century. It is another type of critical angle refractometer.
Working: A glass prism with a high refractive index comprises two planar polar faces which are perpendicular to one another and are placed such that one of them is vertical and the other is horizontal. The substance of which the refractive index is to be calculated is placed on the horizontal surface. A beam of monochromatic light is incident in a direction parallel to the horizontal surface so that the light entering the prism is at critical angle α. Finally, it emerges from the prism at an angle β.
The angle of refraction is measured with the help of telescope. The emergent beam forms a boundary of dark and bright side by the ray that grazes the prism surface, and the sharp boundary is observed with a telescope attached to a divided circle. If the refractive index of the glass nG is known and angle i (β) is measured, then n, the refractive index of the liquid sample can be calculated.
n= √( n^2G – sin^2i)
FACTORS AFFECTING REFRACTIVE INDEX
Various factors that affect refractive index measurement are
1. Temperature: Temperature influences the refractive index of a medium primarily because of the accompanying change in density.
2. Wavelength of light used: The refractive index of a transparent medium gradually decreases with increasing wavelength; this effect is referred to as normal dispersion.
3. Pressure: The refractive index of a substance increases with pressure because of the accompanying rise in density. The effect is most pronounced in gases and it is yet smaller for solids.
APPLICATION OF REFRACTIVE INDEX
1. It is mainly used to identify a particular substance, confirm its purity or measure its concentration. Generally, it is used to measure the concentration of a solute in an aqueous solution. It can also be used in the determination of drug concentration in the pharmaceutical industry.
2. It is used to calculate the focal power of the lenses and the dispersive power of the prism.
3. It also applies to the estimation of the thermophysical properties of hydrocarbons and petroleum mixtures.
4. It is used in the examination of organic compounds (oils, solvents, etc.), solutions, food products, serum protein concentration.
5. In medicine, a refractometer is used to measure total plasma protein in a blood sample and a specific gravity of urine.
6. It is used to determine the amount of sugar in sugar solutions and in general to determine total solids in fruits juices, tomato products, honey, syrups and soda water.
DIELECTRIC CONSTANT
Dielectric constant or relative permittivity (εr) of a solvent is measure of its polarity. The higher the dielectric constant of a solvent of a solvent, the more polar it is. It is a physical property.
Dielectric Constant (ε0) is the ratio between the permittivity of the medium (ε) to the permittivity of free space (ε0) Or is the ratio between force acting between two opposite charges at free space to force acting between two opposite charges at any medium.
The characteristics of a dielectric material are determined by the dielectric constant.
K= F0 /F = (1/4πr^2 × q1*q2/ε0) / (1/4πr^2 × q1*q2/ε)
= ε/ε0 = εr
The dielectric constant is effected both by the attractive forces that exist between the atoms and also the molecules. The solvent with a high dielectric constant makes it possible to more effectively screen the attractive and repulsive force between the ions.
MEASUREMENT
It is measured by measuring the effect of the intervening solvent on the electric field between two opposite charged particles. first the capacitance of a test capacitor, C0, is measured with vacuum between its plates. Then, using the same capacitor and distance between its plates, the capacitance C with a dielectric between the plates is measured. Then, the dielectric constant is obtained which is the ratio of the capacitance (C) of a capacitor completely filled with the dielectric to the capacitance (Co) of the capacitor filled with space or vacuum.
ε= C/C0
and C0 = ε0 A/t
Where
C= capacitance using the material as the dielectric in the capacitor,
C0= capacitance using vacuum as the dielectric
ε0 = permittivity of free space (8.85 x 10-12 F/m)
A = Area of the plate/ sample cross section area
t = Thickness of the sample
It has no dimension.
FACTORS AFFECTING DIELECTRIC CONSTANT
- Frequency: The frequency of the applied voltage is one of the factors affecting dielectric constant. As the frequency of the applied voltage increases, the value of the dielectric constant becomes non-linear.
- Applied voltage: When a direct current voltage is applied, the value of the dielectric constant reduces while applying alternating current voltage would increase the value of the dielectric constant.
- Temperature: When the temperature is low, the alignment of the molecules in the dielectric material is difficult. By increasing the temperature, the dipoles in the dielectric material become dominant resulting in an increase in the dielectric constant. This temperature is known as the transition temperature. If the temperature rises above the transition temperature, then there will be a gradual decrease in the dielectric constant.
APPLICATION OF DIELECTRIC CONSTANT
A major use of dielectric is to manufacture capacitors. These have many uses, including strong energy in the electric field between the plates, filtering out noise from signals as part of resonant circuit and providing a burst of power to another component.
Some application of dielectrics relay on their electrically insulting properties rather that their capacity to store charges. The most obvious of these uses is the insulation of the wires, cables etc.
DIPOLE MOMENT
When non-identical atoms (A and B ) are joined in a covalent bond, the pair of electrons will be attracted more strongly to the atoms that has the higher electronegativity. If A is more electronegative than B, it has a greater tendency to attract electrons. Thus, the shared pair of electron is drawn near A, leaving a positive charge on the B atom the molecule becomes dipolar (A-B+). It is the measurement of polarity.
A dipole moment is a quantity that describes two opposite charges separated by a distance.
The dipole moment, µ, is the product of the magnitude of the separated charge and the distance of the separation.
µ =q x r
where
q is the magnitude of the separated charge and
r is the distance between them.
Greater the charge, larger will be dipole moment. And smaller the distance, larger will be dipole moment.
In SI units, q is expressed in coulombs and r in meters, so µ has the dimensions of C-m. It is expressed in the units of Debye and written as D ( 1 Debye = 1x10-18 e.s.u Cm). Dipole moment is vector quantity.
MEASUREMENT
When a solution of polar molecules is placed between two plates having opposite charge, they align themselves along the direction of the field. This process consumes energy that is returned to the electric circuit when the field is switched off, an effect known as electrical capacitance. The measurement of the capacitance of a gas or a solution serves to determine the magnitude of the dipole moment of a substance.
FACTORS AFFECTING DIPOLE MOMENT
- Polarity of molecule: It is the measure of polarity. More stronger the polarity, more is the dipole moment.
- Magnitude of charge: Larger the electronegativity, more is the dipole moment.
- Geometry of molecule: In more complex molecules with polar covalent bonds, the three-dimensional geometry and the compound’s symmetry determine whether there is a net dipole moment.
- Mathematically, dipole moments are vectors; they possess both a magnitude and a direction. The dipole moment of a molecule is therefore the vector sum of the dipole moments of the individual bonds in the molecule. If the individual bond dipole moments cancel one another, there is no net dipole moment. Such is the case for CO2, a linear molecule. Each C–O bond in CO2 is polar, yet experiments show that the CO2 molecule has no dipole moment. Because the two C–O bond dipoles in CO2 are equal in magnitude and oriented at 180° to each other, they cancel. As a result, the CO2 molecule has no net dipole moment. In contrast, the H2O molecule is not linear, it is bent in three-dimensional space, so the dipole moments do not cancel each other. Thus a molecule such as H2O has a net dipole moment.
APPLICATION OF DIPOLE MOMENT
- For predicting the nature of the molecules: The measurement of the dipole moment helps to distinguish polar and non-polar molecules. Molecules with a zero dipole moment are not polar while the molecule with a specific dipole moment is polar in nature.
- Degree of polarity: The measurement of the dipole moment gives an idea of the degree of polarity in a diatomic molecule. The greater the dipole moment, the greater the polarity in such a molecule.
- Shape of Molecules: The dipole moment is used to find the shape of molecules. If a molecule has a specific dipole moment, its shape will not be symmetrical, it may be curved or angular and the molecule at zero dipole will be symmetric and has a linear form.
- Predict nature of bond: It is possible to predict the nature of chemical bond formed depending upon the electronegativities of atoms involved in a molecule. The bond will be highly polar if the electronegativities of two atoms are large.
DISSOCIATION CONSTANT
Dissociation constant (Kd) is the tendency of a substance AxBy to reversibly dissociate (separate) in a solution into smaller compound A and B is shown as
AxBy = xA + yB
Dissociation constant (Kd) = [A]^x[B]^y/ [AxBy]
Where
[A] is the molar concentration of A
[B] is molar concentration of B and
[AxBy] is the molar concentration of AxBy.
Dissociation constant is defined as tendency of particular substance in solution is dissociated into ions. It is equal to the product of the respective ion concentrations divided by the concentration of the non-ions molecule. The acid dissociation constant (ka) is a quantitative measure of the strength of acid in the solution.
HA = A- + H+;
For this reaction Acid dissociation constant ka will be:
Ka = [A-][H+]/ [HA]
MEASUREMENT
pKa can be measured by using any one of the following methods:
Potentiometric titration,
Spectrophotometric methods,
NMR titration,
Liquid chromatography (LC),
Capillary electrophoresis (CE)
Combined methods,
Computational methods
Of which potentiometer/half neutralisation is more common.
When a strong acid is titrated against strong base it forms a mixture of acid and its salt. The mixture acts as a buffer solution. During the course of titration acid concentration decreases and salt concentration goes on increasing. During any stage of titration the pH of buffer solution is explained by the Henderson –Hasselbalch equation.1
pH = pKa + log [Salt]/ [Acid] …… (1)
Where,
pKa = -logKa and Ka = dissociation constant of the acid
[Salt] = concentration of salt
[Acid] = concentration of acid
At the half neutralization point
[Salt] = [Acid]
And equation (1) above gives pH = pKa
FACTORS AFFECTING DISSOCIATION CONSTANT
Nature of electrolyte: Stronger the electrolyte, more is the dissociation and vice versa.
Nature of solvent: More is the dielectric constant of the solvent, more is the dissociation.
Concentration: Lesser the concentration, greater is the ionisation.
Temperature: Higher the temperature, more is the ionisation.
APPLICATION OF DISSOCIATION CONSTANT
The acid-base dissociation constant (pKa) of a drug is a key physic-chemical parameter influencing many biopharmaceutical characteristics.
The knowledge of the pKa values is important for for the quantitative evaluation of systems involving acid-base equilibria in solution.
Dissociation constant (Ka) is also essential for working with buffers. The design of these solutions depends on a knowledge of the pKa value of their components.
Other application of Ka is with pH indicators. A pH indicator is a weak acid or a weak base which changes color in the transition pH range , is about pKa 1.
OPTICAL ROTATION
Many substances process the inherent property to rotate the plane of incident polarized light. This is called optical activity.Optical rotation is the angle at which the plane of polarization is rotated when the polarized light passes through a layer of one liquid.
The optically active substances are described as dextrorotatory or levorotatory depending on whether the plane of polarization is rotated clockwise or anticlockwise.
Dextro rotator or right handed: The substance which rotates plane of polarization of light towards right or in clockwise direction are called dextrorotatory or right handed. The dextrorotation is designated(+)
Laevo rotator or left handed: The substance which rotates plane of polarization of light towards left or in anti-clockwise directions are called laevo rotator or left handed. Levorotation is designated(-)
In the International Pharmacopoeia, optical rotation (α) is expressed in angular degrees. In the SI, the optical rotation angle is expressed in radians (rad).
Where [α] = optical rotation (Unit – degrees cm3/dm g)
λ = wavelength in nanometer
α = measured angle of rotation
T = temperature in degrees
l = path length in dm
c = concentration of sample in g/ml
MEASUREMENT
Polarimeters measure the degree of rotation of polarized light as it passes through an optically active material.
INSTRUMENTATION
A polarimeter consists of a:
- Polarized light source
- ( sodium vapour lamp),
- Polarizer
- Analyzer and
- Sample tube
- Detector
PROCEDURE
When light is passed through a polarizer only light oscillating in one plane will leave the polarizer. The plane-polarized light is introduced to a tube containing a solution with the substance to be measured. If the substance is optical inactive, the plane of the polarized light will not change in orientation and the observer will read an angle of [α]=00. If the compound in the polarimetry cell was optical active, the plane of the light would be rotated on its way throught the tube either clockwise or counter clockwise direction depending on the nature of the compound.
For clockwise direction the rotation (in degrees) is defined as positive (“+”) and called dextrorotatory. In contrast, the counter clockwise is defined as negative (“ – “ ) and called levorotatory.
FACTORS AFFECTING OPTICAL ROTATION
- For a given compound, the extent and direction of this rotation depends on the orientation of the molecules in relation to the beam.
- Direction of beam onto the molecule.
- Physical properties of crystal it is directly proportional.
- Thickness of crystal.
- Mass density of the crystal or concentration in the case of solutions.
- Wavelength of light used
- The temperature of the solution.
APPLICATION OF OPTICAL ROTATION
- Identification and determination of optically active compounds
- Sugar industry: Polarimeters are used in the sugar industry to determine the quality of juice from sugarcane and refined sucrose. Often, sugar refineries use a modified polarimeter with a flow cell called a saccharimeter.
- Chemical industry: Many chemical exhibit specific rotation as a unique property that can be used to distinguish it.
- Food, beverage and pharmaceutical industries: The concentration and purity measurements of the samples which have specific rotations can be calculated with a polarimeter . These include: Steroids, Diuretics, Antibiotics, Narcotics, Vitamins, Analgesics.
- If the specific rotation of a sample is known its concentration in the solution can be estimated.
- If the concentration of the material in the sample is known then its specific rotation can be determined.